
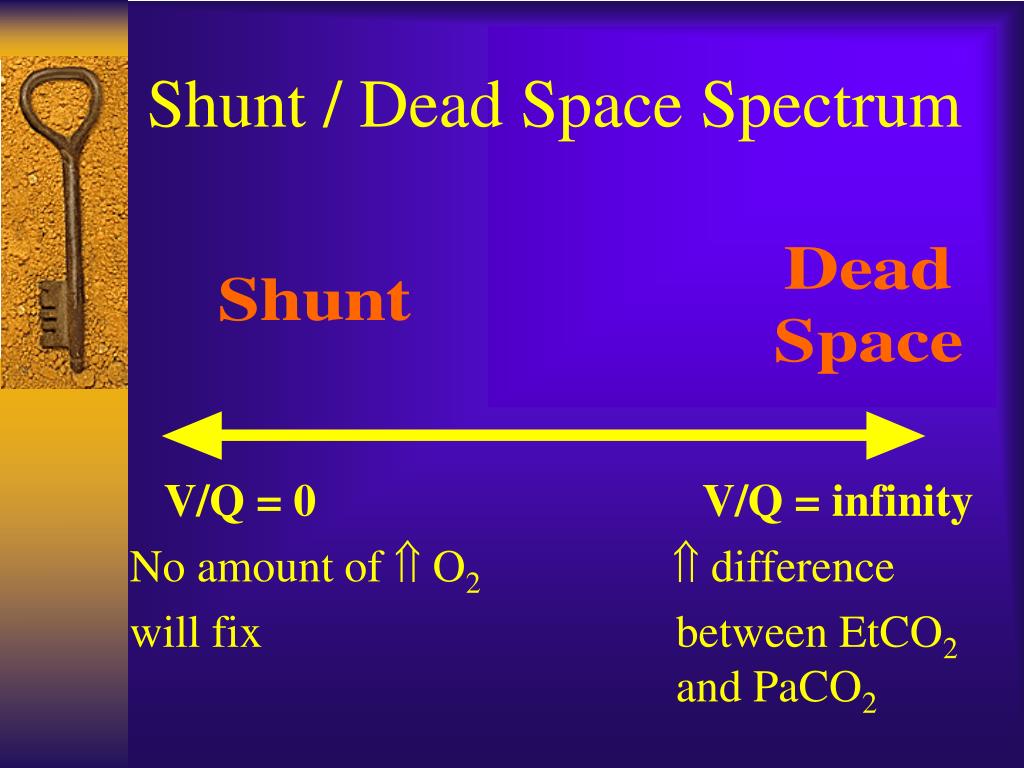
ģ0 patients admitted to Danderyd Hospital in Stockholm, Sweden who were ≥18 years old and PCR-positive for SARS-CoV-2 were recruited between November and December 2020. Using bedside measurement of exhaled partial pressure of oxygen ( P O 2) and carbon dioxide ( P CO 2) combined with arterial blood gas measurements, we measured alveolar–arterial partial pressure differences for both oxygen ( P A−aO 2) and carbon dioxide ( P a−ACO 2), from which both intrapulmonary shunt and alveolar dead space values were then determined using a novel computational model. We hypothesised that in early COVID-19 pneumonitis, hypoxaemia is associated with both increased intrapulmonary shunt and increased alveolar dead space. Pulmonary vessel microembolism reduces capillary blood flow, thus generating areas of high V′ A/ Q′ and promoting increased alveolar dead space. However, pathology reports from COVID-19 infected lungs also frequently demonstrate pulmonary vasculature involvement, including severe endothelial injury, widespread thrombosis with microangiopathy and new vessel growth. The hypoxaemia is likely related to ventilation/perfusion ( V′ A/ Q′) mismatch and in particular to increased intrapulmonary shunt arising from alveolar filling with fluid or cellular debris. Patients with early COVID-19 respiratory failure present with hypoxaemia and hyperventilation. Worldwide there have been more than 6 million deaths. Many have required hospitalisation, with 10–20% of those requiring intensive care, mainly due to respiratory compromise. Since the beginning of 2020, over half a billion people have been diagnosed with coronavirus disease 2019 (COVID-19), a disease caused by infection with the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).


 0 kommentar(er)
0 kommentar(er)
